2020 AND BEYOND:
The evidence supporting the continued role of hydrogel daily disposables in contemporary contact lens practice
Robin Chalmers*, Noel Brennan, David Ruston
Introduction
As the Daily Disposable (DD) contact lens market continues to grow across the world, fitting data shows there continues to be a role for both Silicone Hydrogel (SiHy) and hydrogel materials when best meeting patient needs, especially in relation to comfort and healthy contact lens wear. Euromonitor data indicates that for new fits and refits, 68% of DD fits in 2018 were into hydrogel materials in the United States, a number significantly higher than that in the UK where only 25% of DD fits were into hydrogel matierals.1 This data also supports the established widespread global use of this material, a fact illustrated by 1-DAY ACUVUE® MOIST remaining the number one selling DD contact lens (CL) brand in the world.‡
There is no doubt that SiHys have a higher Dk/t than hydrogels along with the professional belief that this contributes to healthier contact lens wear.2,3 However, we also know that overall lens performance is driven by much more than Dk/t and that comfort, inflammatory events and incidence of Microbial Keratitis (MK) are not improved by increasing Dk/t, especially during daily wear.4-9 Additionally, as recently stated by Orsborn and Dumbleton, there is currently no clear evidence in the literature to support SiHy DD contact lenses being superior to hydrogel DDs with respect to adverse events, wettability or comfort.2
Given the focus on oxygen delivery in professional education, it is possible some eye care professionals (ECPs) feel an expectation to proactively move away from hydrogel materials for both new and existing patients. However, many patients continue to do very well in hydrogel DDs, with one recent study showing higher comfort scores when compared to some SiHy brands.9
To be able to choose between more than one type of CL material appears desirable, so how should ECPs be thinking about hydrogel DDs in their contemporary CL practice? To help answer this question this article reviews the performance of hydrogel DDs, in particular 1-DAY ACUVUE® MOIST, and also reports on more recently published work in this area. A number of commonly held beliefs about hydrogel CL materials are summarised in Table 1, with the evidence to contradict each belief being discussed in more detail throughout this article.
Table 1: Summary of the effect of photochromic spectacle lenses on visual performance.
| Area of potential concern | Perception | Evidence |
| Comfort | SiHy DDs are always as or more comfortable than hydrogel DDs | Multiple studies show no clear evidence for this,9-11 and one study shows a hydrogel DD CL more comfortable than some SiHy DDs.9 Comfort can vary significantly amongst DD SiHy brands.9,12 |
| Corneal oxygenation | Unacceptable levels of corneal swelling in daily wear | Multiple recent investigations show clinically insignificant degrees of corneal edema in daily wear, both centrally and peripherally (maximum difference <1.5%)13-15 |
| Limbal hyperemia | Marked ocular redness due to peripheral hypoxia | Multiple studies show either no or no clinically significant increase (<0.5 grade on 0-4 scale).13,15-17 |
| Neo-vascularization in long-term wearers | A common issue indicating unhealthy CL wear | The authors have not seen any clinical evidence in over 30 years history of the use of mid-water content thin hydrogels. A recent study cited as evidence of an issue,18 defines neo-vascularization as vessel extension of 0.5mm or more when 0.5mm is half of grade 1 (<1mm) on the Efron scale and rated as not clinically significant. |
| Safety | Hydrogel DD lenses are less safe than SiHy DDs, especially if wearer naps or wears overnight. | In major studies,7,19 etafilcon A lenses (1-DAY ACUVUE® MOIST) were associated with no or minimal symptomatic corneal infiltrative events or other serious adverse events, consistent with studies showing less CIEs in hydrogels compared to SiHys. No difference in MK rates between hydrogel and SiHy in daily wear or overnight wear.4,20-22 In fact, etafilcon A shown to be associated with lower risk of MK than other materials.23 No similar historical evidence available for SiHy DD lenses. |
Factors contributing to successful contact lens wear
Comfort, vision and health are often used to describe the main areas of performance deemed most important in relation to achieving successful CL wear. Certainly, it is true that suboptimal performance in the first two aspects, comfort and vision, can result in dropping out of CL wear.24-26 The third factor, health, is often predominantly discussed in terms of the oxygen performance of the lens. This, however, is an over simplification. When considering the ability to wear CLs safely, oxygen performance forms just one part of the overall health and safety profile of the lens, alongside other aspects such as the rate of inflammatory and infective events, all of which will be reviewed here. In fact, as will be shown, the oxygen performance of etafilcon A lenses in daily wear is comparable to SiHy DDs for most lens powers.
Patient experience: comfort and vision
Comfort is the factor most linked to drop out from CL wear,27 which raises the question as to whether differences in performance exist between material groups. It is important to understand that the comfort of a CL is not driven by its oxygen transmissibility,28 and the combined results from multiple studies do not report consistent conclusions for comfort preference of one material type over another.8-10,29-33 In fact, as outlined in the Tear Film and Ocular Surface Society (TFOS) Contact Lens Discomfort report, there are many factors of lens design and how they interact with the ocular surface and tear film that contribute to how the lens feels when worn.28
A recently published study examined the comfort of three SiHy DDs and two hydrogel DDs from three manufacturers. It found one of the hydrogel DDs to be significantly more comfortable than one of the SiHy DD CLs.9 The authors deemed the difference to be clinically significant. The hydrogel lens brand in question, which remains masked in the publication, was shown to deliver comfort levels similar to those experienced by non-lens wearing emmetropes.
Poor vision, primarily in astigmatic or presbyopic patients can lead to drop out.25,26 It is useful to remember that 1-DAY ACUVUE® MOIST benefits from having a full family of designs available to correct vision for these patients. Further, LACREON® Technology permanently embeds a water-holding ingredient (polyvinylpyrollidone, PVP) into 1-DAY ACUVUE® MOIST which results in more stable visual performance between blinks compared to a lens with no wetting agent.34
In terms of comfort and vision performance, evidence suggests that ECPs can continue to include 1-DAY ACUVUE® MOIST in their thinking when considering lens choice for their patients. In fact, in 13 clinical studies posted on www.clinicaltrials.gov, 1-DAY ACUVUE® MOIST Brand contact lenses have never been beaten in comfort in its category,† or in vision.§ The last important CL performance indicator is the topic of overall safety. To examine this area, aspects relating to ocular health and safe wear are reviewed individually, starting with oxygen transmissibility.
Health: corneal oxygenation
There is considerable evidence that 1-DAY ACUVUE® MOIST, being a thin mid-water content lens, does not produce clinically significant levels of corneal oedema during daily wear. The work of Szczotka-Flynn,13 assessed corneal responses during fitting of low to moderate myopes into one hydrogel (etafilcon A) and two common SiHy materials (comfilcon A and Lotrafilcon B) for daily wear. Central corneal thickness measurements were performed with a very precise optical low coherence reflectometry biometer at least 2 hours after waking and at the end of 6-8 hours of wear on day 1 and day 7 of each lens type. Statistically, non-inferiority of the hydrogel material was judged, with respect to the other two lens types, if the corneal swelling was within 1.5%. Knowing that the normal non-lens wearing cornea thins throughout the day, the study results were reassuring in that all three lens types demonstrated no impediment to this normal deswelling process. In particular, the hydrogel lens demonstrated about 0.3% deswelling which was within the non-inferiority margin of the two other silicone hydrogel lens types as seen in Figure 1. Peripheral hypoxic stress was quantified by assessing limbal hyperemia. Consistent with the corneal swelling data, limbal hyperemia with all lenses was negligible and non-inferiority assumptions were met between the hydrogel lens and the two other lens types.
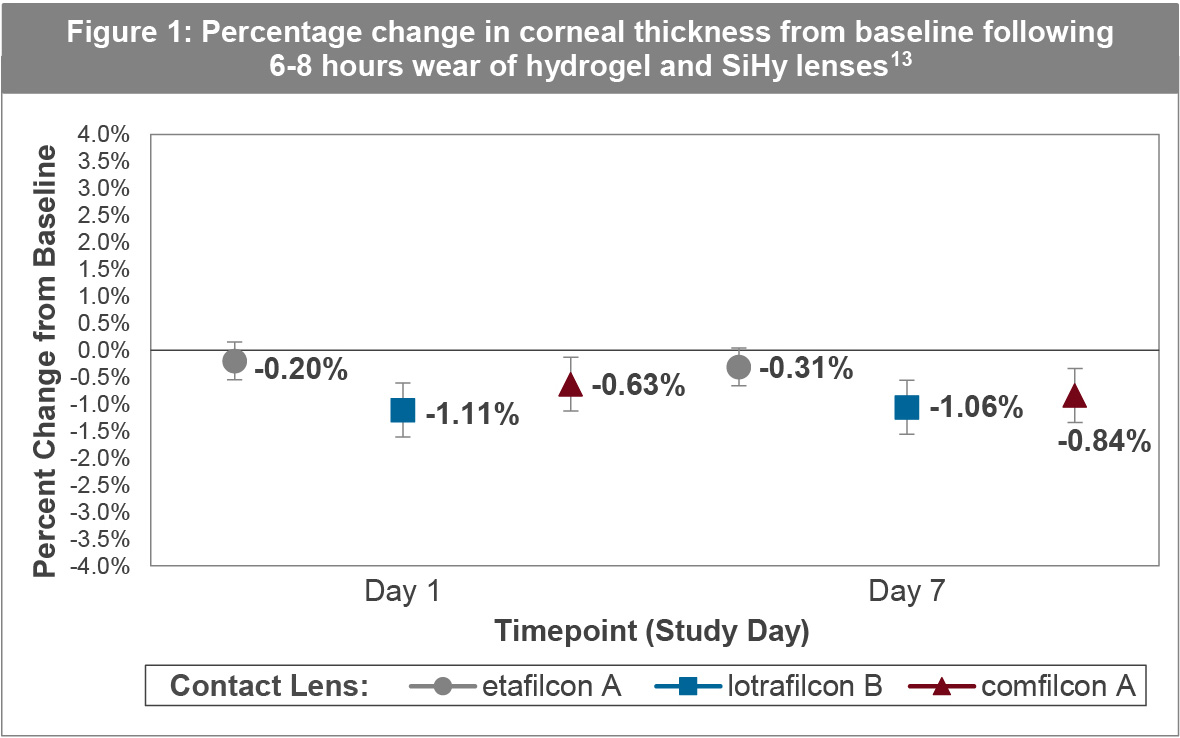
These findings are supported by Moezzi et al,14 who measured central and peripheral corneal swelling in 1-DAY ACUVUE® MOIST after 8 hours compared to no-lens wear in 24 subjects. Central swelling was measured using optical pachymetry, with mean swelling for 1-DAY ACUVUE® MOIST after 8 hours wear being just 0.13%.14 After 8 hours of open-eye wear, central and peripheral corneal swelling along the horizontal meridian with 1-DAY ACUVUE® MOIST was <1.5%, clinically equivalent to that observed with no lens. Conjunctival hyperemia was also clinically unremarkable under both conditions.
A further study by the same team examined physiological response and clinical performance in 39 subjects switched from their habitual SiHy CLs to 1-DAY ACUVUE® MOIST MULTIFOCAL.15 Ocular physiology was assessed at baseline and at 4 weeks, after 6 hours of open-eye wear. When compared to baseline SiHy use, the corneal thickness for each of four corneal locations at day 28 was clinically equivalent (Figure 2). The result was true for both myopes and hyperopes alike.15
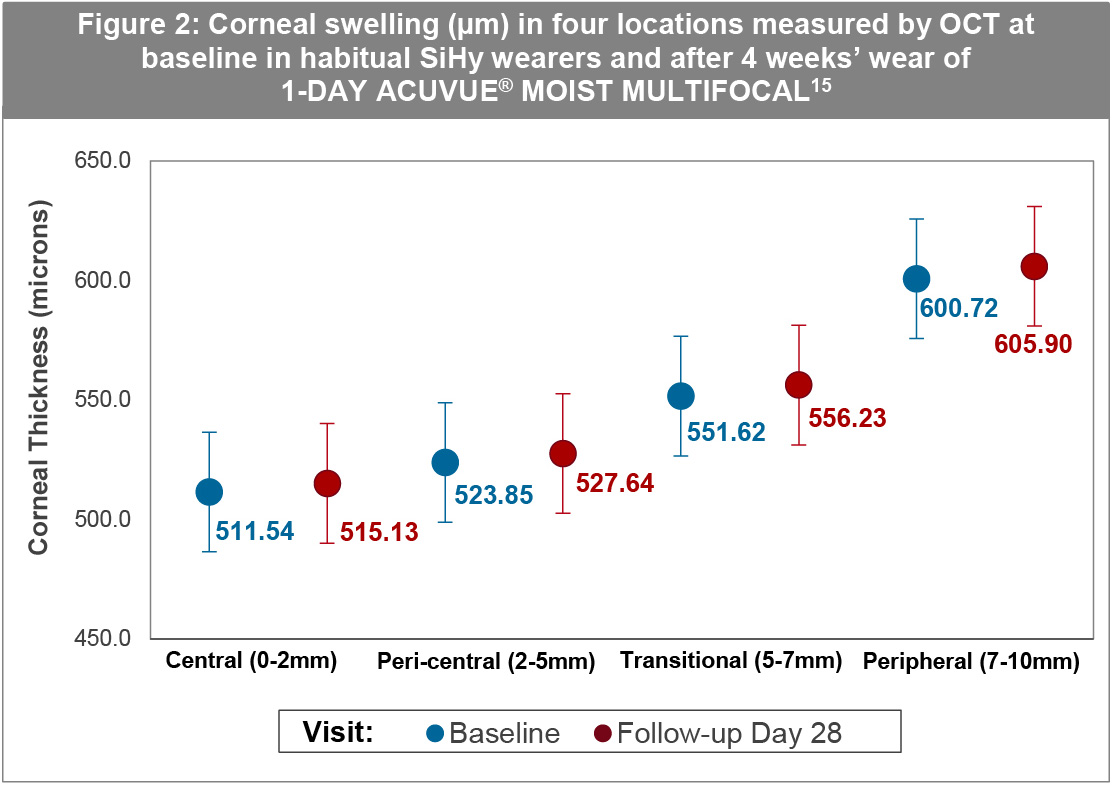
While there might be concern that the results in these studies do not reflect the level of corneal oedema after a full day’s wear as the measurements were taken after 6-8 hours of wear, the benchmark work of Holden et al shows that when subjects are exposed to the levels of oxygen present (as under the contact lenses evaluated) corneal swelling peaks after 2 hours and plateaus thereafter.35 Thus, the time period over which these investigations into corneal oedema were carried out is more than sufficient.
Health: limbal hyperemia and neo-vascularization
A further important indicator of hypoxic stress is limbal hyperemia. The same study discussed above also evaluated this clinical sign. The levels of mean limbal (Figure 3) and bulbar hyperemia at day 28 compared to the habitual SiHy lens baseline were equivalent.15 Again, the same was true in both hyperopes and myopes. The levels of hyperemia observed were consistent with that seen in non-lens wearing adults (≤Grade 1). All of the results from this study, concerning the physiological performance are useful to bear in mind when considering whether to recommend 1-DAY ACUVUE® MOIST MULTIFOCAL to existing SiHy wearers in the presbyopic age group.
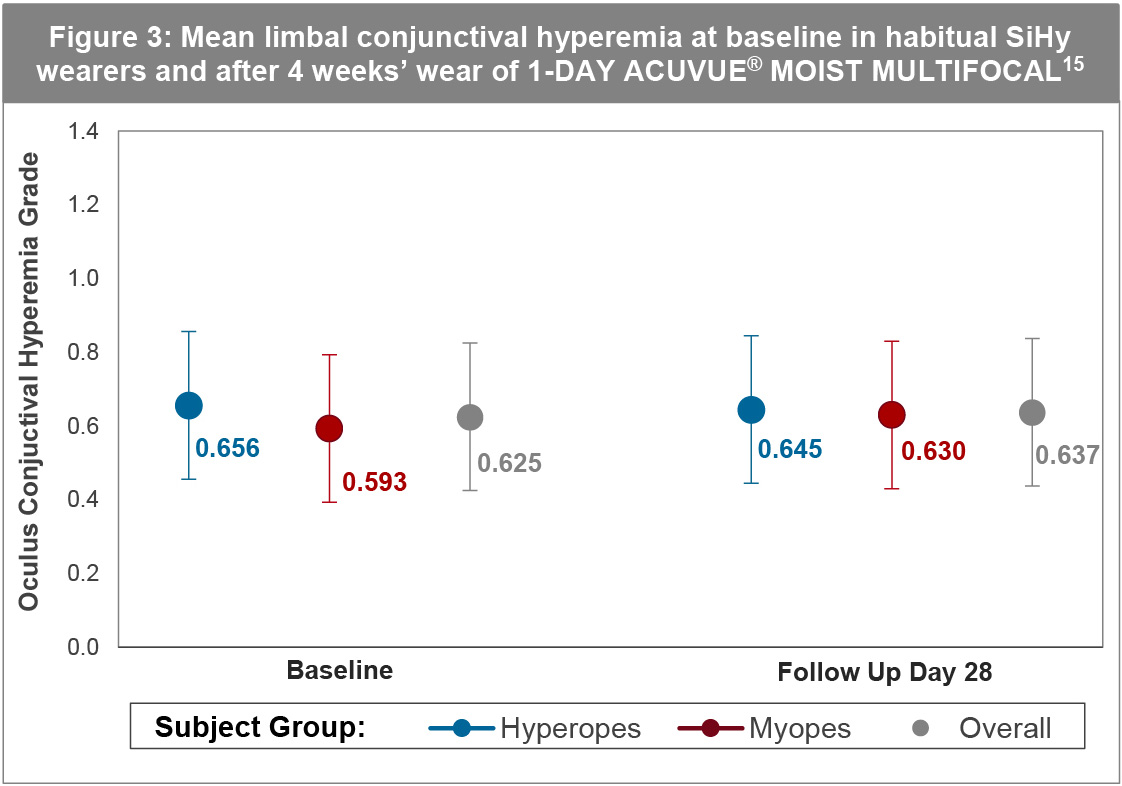
It is true that some studies report higher limbal hyperemia grades resulting from hydrogel compared to SiHy wear, although the differences are so small they are often concluded to not be clinically significant.15-17 One such example of this was seen with a comparison of three ACUVUE® Brand contact lenses, 1-DAY ACUVUE® MOIST, and two SiHys, 1-DAY ACUVUE® TruEye® and ACUVUE® OASYS with HYDRACLEAR® PLUS, all worn on a daily disposable basis.16 The study investigated the physiological response during three months of daily wear of at least 8 hours a day. While the SiHy lenses did result in statistically lower limbal hyperemia scores, the difference was 0.2 units on a 0-4 grading scale. It is generally accepted that a difference of 0.25, or even 0.50 units, represents clinical significance. No other ocular physiological variable – including vascularization – showed significant differences. The authors have not seen any clinical evidence of neovascularization in over 30 years history of the use of mid-water content thin hydrogels. A recent study cited as evidence of an issue,18 defines neo-vascularization as vessel extension of 0.5mm or more when 0.5mm is half of grade 1 (<1mm) on the Efron scale and rated as not clinically significant.
Health: corneal infiltrative events
Corneal infiltrative events (CIEs) are an inflammatory response of the cornea and range from being asymptomatic,
to presenting with signs and symptoms. The TEMPO study measured the incidence of adverse events by post market surveillance registry of wearers fitted with 1-DAY ACUVUE® MOIST.7^ This 12-month observational study of 570 patients (equivalent to 471 patient years of lens wear), found there were no symptomatic CIEs or other serious adverse events (0.0%/year) and only three non-serious events (0.6%/year). However, serious adverse events have been reported at very low rates outside of this study.^
^This observational/surveillance registry relied on patient reports of symptomatic adverse events that led them to seek clinical care. The results should be considered in conjunction with other clinical results on the safety and efficacy of 1-DAY ACUVUE® MOIST, which also generally show low rates of such events. It should be noted that although no symptomatic infiltrative events were reported in the TEMPO study, such events can occur with DD lenses, including 1-DAY ACUVUE® MOIST, as noted in the product labelling.
In addition to TEMPO, an independent, retrospective analysis looked at 28 lens/solution combinations, each tested on approximately 40 patients wearing lenses on a daily wear basis for a 3-month period.19 Lenses were frequent replacement and DDs, hydrogels and SiHys. Solutions included hydrogen peroxide and multipurpose. The overall rate of adverse events was 3.6 events per 100 participant-months. Rates were low for the four DD lenses tested and significantly lower in DDs compared with reusable daily wear lenses (3.1 vs 10.9).19 The only lens in the study that did not have any reported adverse events was 1-DAY ACUVUE® MOIST, although the study did not show statistically significant differences between the DDs evaluated.
Additional analysis of the TEMPO study has since explored the influence of age on performance outcomes.36 86 wearers aged over 40 completed the registry, with 76% new to DDs and 8% new to CLs completely. Existing CL wearers older than 40 years experienced many benefits from being refitted with 1-DAY ACUVUE® MOIST. Changing to the hydrogel DD significantly improved their overall opinion of CLs, improved dry eye symptoms as quantified by the CLDEQ-8 questionnaire,37 and maintained average and comfortable wear time. When the younger age of the sample was analysed using data from subjects less than 18 years old, 1-DAY ACUVUE® MOIST was the only CL to have shown zero symptomatic events in teens in this 1-year observational study.38
Microbial keratitis (MK)
The sight-threatening nature of MK makes this a rare, but serious, adverse event a very important factor to consider. The introduction of SiHy materials has not resulted in a lower incidence of MK.4,21,39 A two-year case control study at Moorfields Eye Hospital in the UK, and a one-year national surveillance study in Australia and New Zealand identified new cases of CL-related MK in wearers using DD and other soft contact lenses.23 Self-administered (cases) or telephone-administered (controls) questionnaires were used to identify potential risk factors for MK, expressed as an odds ratio (OR). A total of 963 DD wearers were identified, from which 67 MK cases and 374 controls were sampled. Independent risk factors for all and for moderate/severe MK were: wearing CL every day vs less frequent use (OR 10.4x), any overnight wear (OR 1.8x), less frequent hand washing (OR 1.8x) and smoking (OR 1.3x).
But the study also found that risk varied with DD lens type and it would seem that certain DDs had lower rates. Using 1-DAY ACUVUE® as the referent (reference standard), the other two DDs had ORs of 2.06 and 3.98, indicating greater risk.23 The authors observe that ‘it is unclear why certain DDs are protective for the risk of infection,’ and that ‘some types of DD are more difficult to remove than others, which may increase the risk of mechanical complications.’ They conclude that lens type, material properties and design are likely to play a role in the aetiology of MK in the DD modality.
Additional attributes of 1-DAY ACUVUE® MOIST
The unique properties of 1-DAY ACUVUE® MOIST contribute to patient comfort. LACREON® Technology permanently embeds a water-holding ingredient, PVP, and enhances moisture retention and reduces friction.40** For the 1-DAY ACUVUE® MOIST family the highest concentrations of PVP are at the lens surfaces (within 10µm),41 where the eye interacts with the lens and thereby benefits most. The result is a long-lasting ‘cushion’ of moisture from the core to the surface.42,43
**The release profile for PVP in 1-DAY ACUVUE® MOIST over 24 hours demonstrated no detectable amount of PVP released throughout the 24-hour simulated wear period.
1-DAY ACUVUE MOIST also helps maintain lysozyme in its natural state, selectively and rapidly attracting asignificant amount of the enzyme. This has been shown in vitro to help maintain low levels of inflammatory biomarkers that can lead to irritation.44~ Other attributes of the 1-DAY ACUVUE® MOIST family include low modulus, the INVISIBLE EDGE® design, Class 2 UV-Blocking ‡**and comprehensive parameters across the family of lenses.45-47#
The 1-DAY ACUVUE® MOIST family is also an excellent option for the significant numbers of CL wearers (52%) who say their eyes feel sensitive,45 possibly describing irritation, redness, stinging.46

Conclusions
- This article reviews the evidence for the comfort, vision and the safety of hydrogel DDs. Over time, multiple studies have consistently reported high levels of performance across all these areas, for hydrogel DDs in general, and for 1-DAY ACUVUE® MOIST in particular.
It remains true that there are situations where consideration of oxygen access is necessary, for example, those patients with higher spherical prescriptions or those requiring certain thicker toric lens designs. There is no doubt that some SiHy brands can offer improved comfort and vision due to lower coefficient of friction and tear lens stability.12 However, hydrogel DD lenses are still an excellent choice for new wearers, and for existing wearers who exhibit no subjective or clinical issues. In the authors’ opinion, many existing hydrogel wearers will not benefit from being automatically refitted with silicone hydrogel DDs.
1-DAY ACUVUE® MOIST remains the number 1 selling DD CL brand in the world,‡ and for good reason. It has an excellent safety profile,7^ is the only DD lens shown to have zero adverse events in teenagers in the year-long TEMPO observational study,38 in 14 clinical studies. 1-DAY ACUVUE® Brand family of contact lenses have never been beated in comfort when compared to other hydrogel daily disposable contact lenses, and in 20 clinical studies has never been beaten in vision, offer a full family of lenses to satisfy most pateints' needs. and created clinically insignificant corneal oedema and hyperaemia during daily wear.13-17
The evidence is very clear on the benefits of the single use CL modality, however the ECP still has an important role in selecting the optimal material type for the patient’s individual eyes factoring in patient history, age, tear quality and lifestyle needs. Hydrogel DDs continue to play an important role as part of the portfolio of products that practitioners can offer. To suggest otherwise fails to acknowledge the considerable evidence base and would deprive ECPs of an important option for their use in contemporary clinical practice.
Dr Robin Chalmers is a Clinical Trial Consultant based in Atlanta, Georgia. Dr Noel Brennan is Clinical Research Fellow and Global Platform Lead Myopia Control at Johnson & Johnson Vision Care, Inc. David Ruston is Director Global Education at Johnson & Johnson Vision Care, Inc.
*Dr. Robin Chalmers is a paid consultant to Johnson & Johnson Vision Care, Inc

