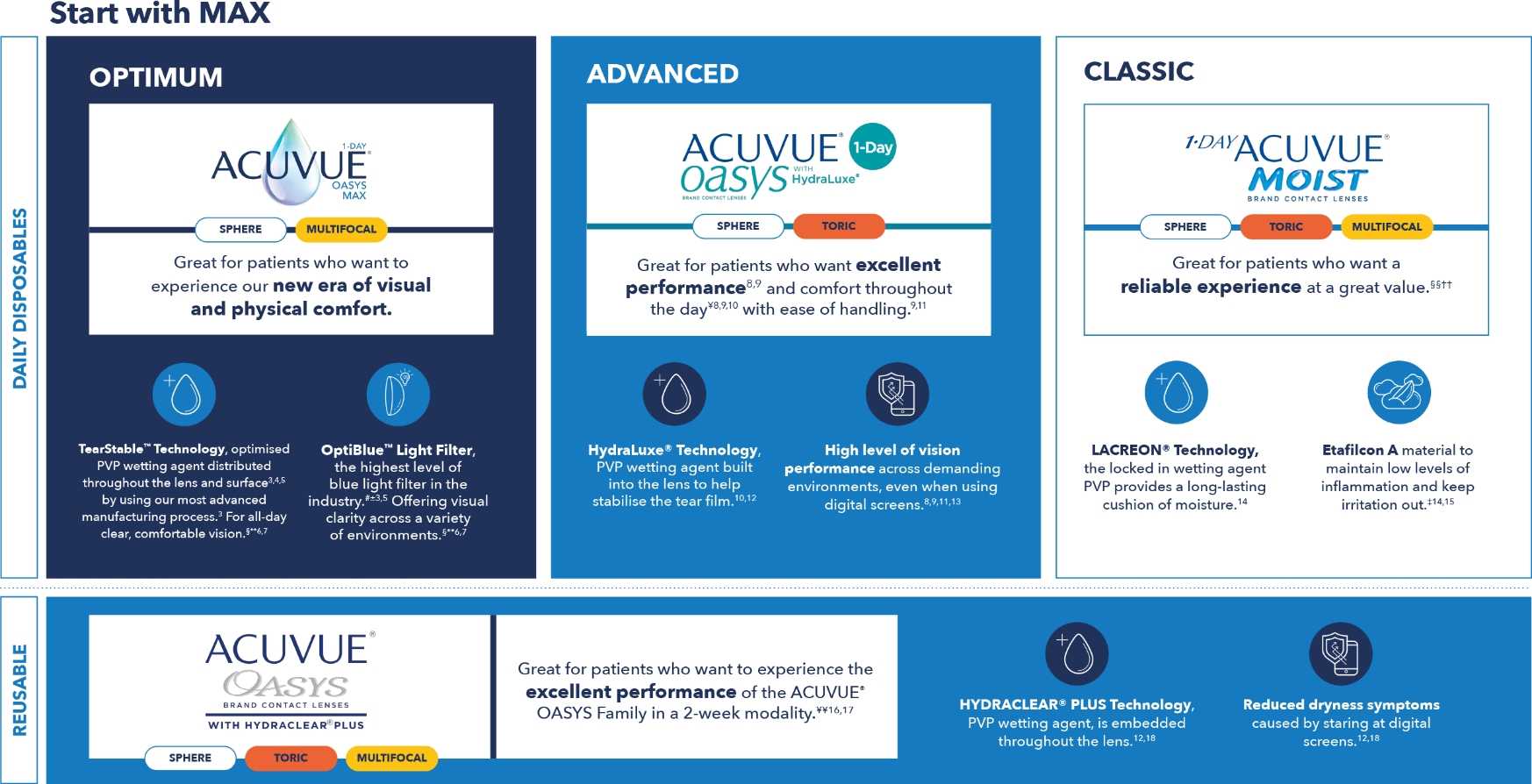
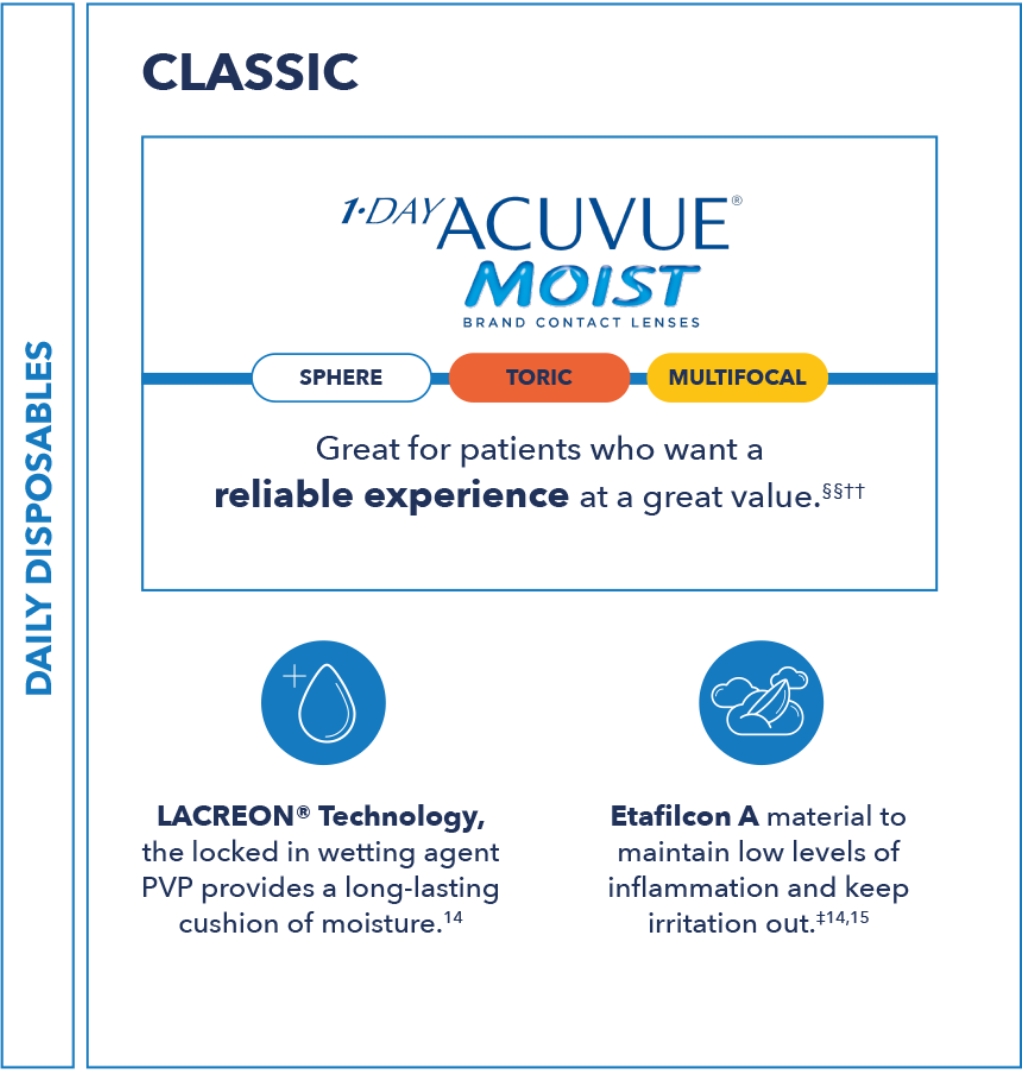
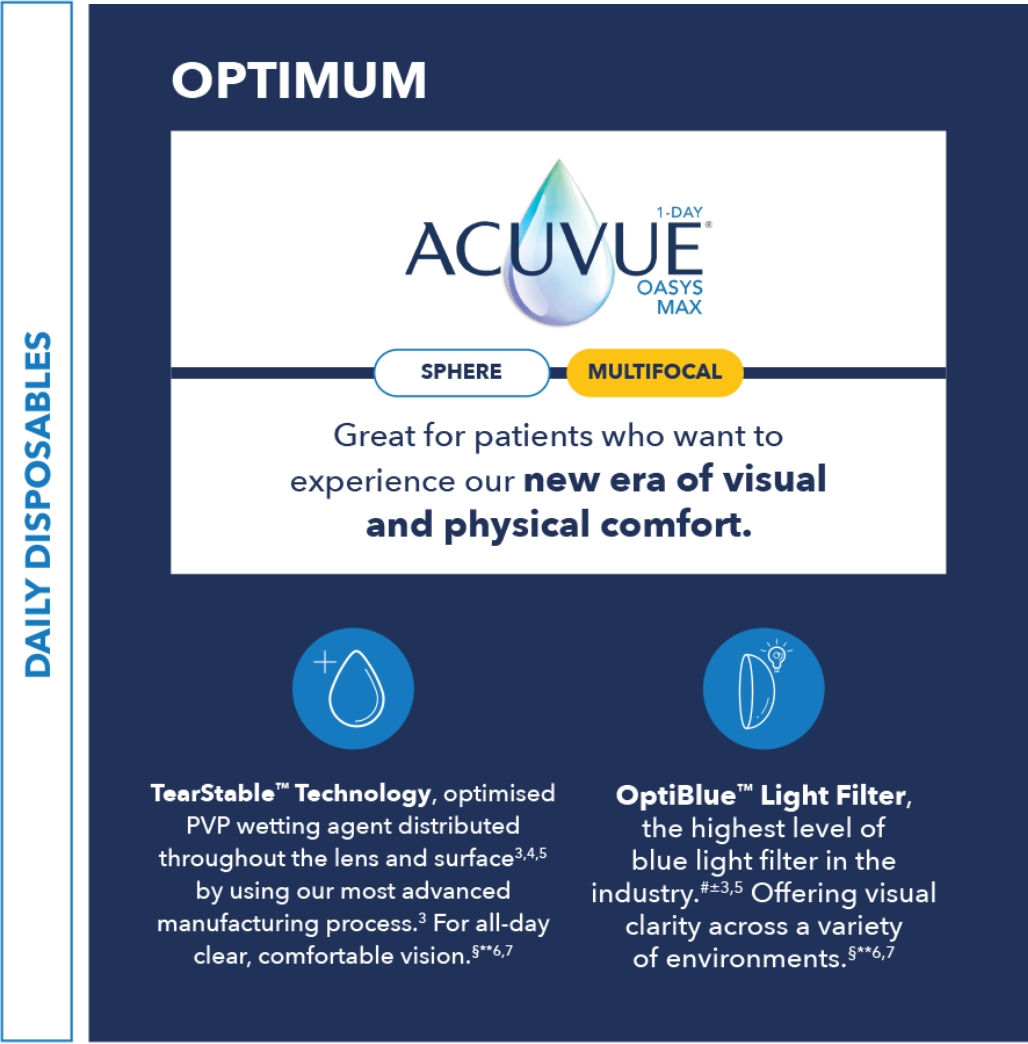
This framework is a quick tool to understand and differentiate ACUVUE® contact lenses.

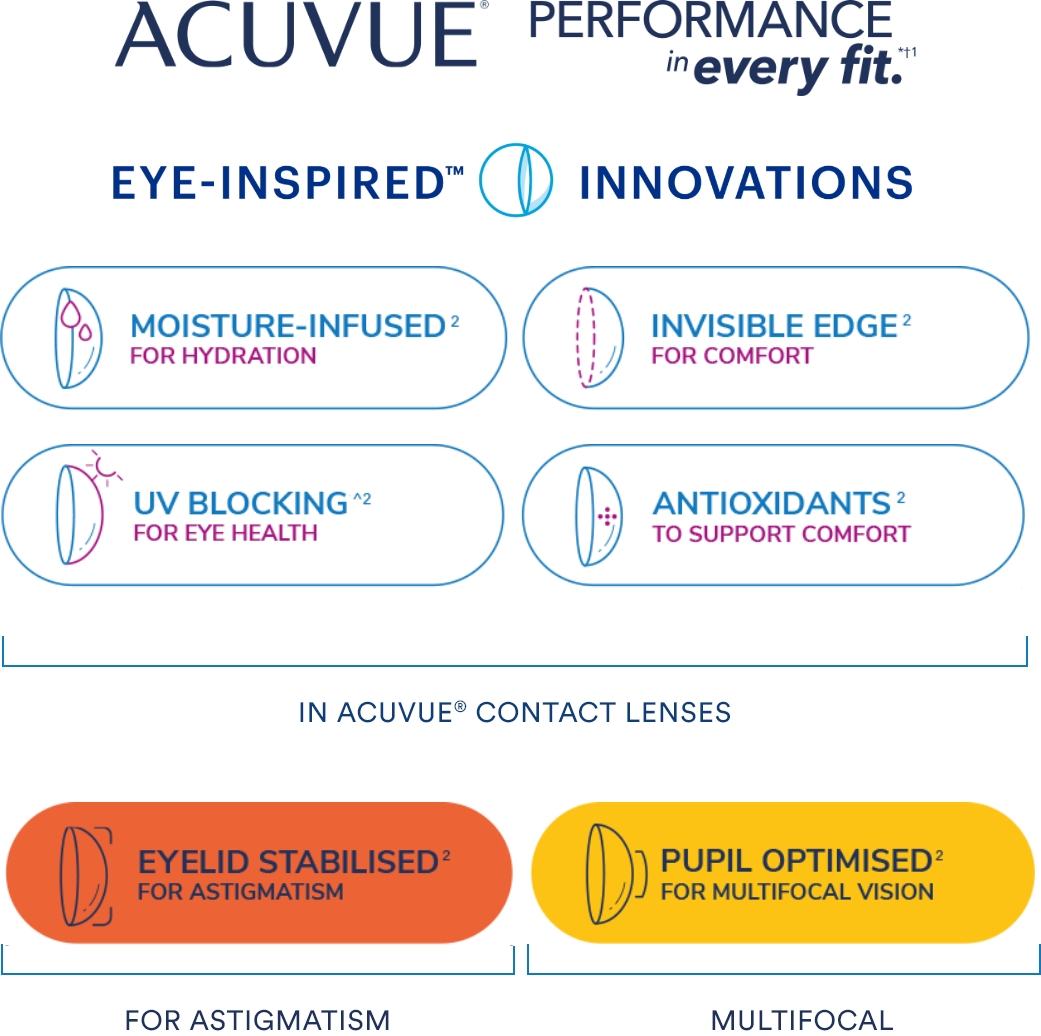
This framework is a representation of the ACUVUE® Portfolio.
Download a copy of the ACUVUE® framework
* 'Excellent Vision' claim does not include ACUVUE® Soft Contact Lenses indicated for myopia management.
† Subjective Comfort Data has not been collected for the ACUVUE® drug-eluting [medication releasing] contact lenses.
^ All ACUVUE® Contact Lenses have Class 1 or Class 2 UV-blocking to help provide protection against transmission of harmful UV radiation to the cornea and into the eye. UV-absorbing contact lenses are NOT substitutes for protective UV-absorbing eyewear such as UV-absorbing goggles or sunglasses because they do not completely cover the eye and surrounding area. UV transmission measured with -1.00 lens.
§ n≥449.
**n=378.
# Filtering of HEV light by contact lenses has not been demonstrated to confer any systemic and/or ocular health benefit to the user. The Eye Care Professional should be consulted for more information.
± Versus publicly available information for standard daily use contact lenses as of December 2023.
¥ Designed to support the functions of the tear film.
‡ Based on in-vitro data, clinical studies have not been done directly linking differences in lysozyme profile with specific clinical benefits.
§§ ClinicalTrials.gov is a website maintained by the NIH. The 14 clinical studies evaluated subjective comfort as a primary or secondary endpoint for 1-DAY ACUVUE® MOIST Brand family (spherical, astigmatism, and multifocal) contact lenses vs. competitors' products. Review conducted as of April 30, 2024.
†† ClinicalTrials.gov is a website maintained by the NIH. The 20 clinical studies evaluated objective and subjective vision as a primary or secondary endpoint for 1-DAY ACUVUE® MOIST Brand family (spherical, astigmatism, and multifocal) contact lenses vs. competitors' products. Review conducted as of April 30, 2024.
¥¥ ClinicalTrials.gov is a website maintained by the NIH. The 22 clinical studies evaluated subjective comfort as a primary or secondary endpoint for ACUVUE® OASYS Brand 2-week family contact lenses vs. competitors' products. Review conducted as of April 30, 2024.
1. JJV Data on File 2022: Master Brand Claims on Clinical Performance and Overall Material Properties for ACUVUE® Brand Soft Contact Lenses.
2. JJV Data on File 2020. ACUVUE® Brand - Eye-Inspired® Innovations.
3. JJV Data on File 2022. TearStable™ Technology Definition.
4. JJV Data on File 2022. Effect on Tear Film and Evaluation of Visual Artifacts of ACUVUE® OASYS MAX 1-Day Family with TearStable™ Technology.
5. JJV Data on File 2022. Material Properties: 1-DAY ACUVUE® MOIST, 1-DAY ACUVUE® TruEye®, ACUVUE® OASYS 1-Day with HydraLuxe® Technology and ACUVUE® OASYS MAX 1-Day with TearStable™ Technology Brand Contact Lenses and other daily disposable contact lens brands.
6. JJV Data on File, 2022. CSM Subjective Responses ACUVUE® OASYS MAX 1-Day Contact Lenses- Retrospective Meta-analysis.
7. JJV Data on File 2022. Subjective Stand-Alone Claims for ACUVUE® OASYS MAX 1-Day MULTIFOCAL Contact Lenses - Exploratory Meta-analysis.
8. JJV Data on File 2016. Single-masked, 2-visit, bilateral wear, single arm 1-week daily wear dispensing study of 162 US habitual toric soft contact lens wearers in both eyes, with a spherical distance refraction of -1.50D to -4.00D (inclusive), cylinder correction of 0.75D to 1.50D after vertex correction (inclusive), and refractive cylinder axis within 180±15 degrees or 90±15 degrees.
9. JJV Data on File 2015. 1-week dispensing evaluation, daily wear study, n=119 soft contact lens wearers in the United States.
10. JJV Data on File 2021. HydraLuxe® Technology Definition.
11. JJV Data on File 2016. Design Enhancements and Resultant Benefits of ACUVUE® OASYS Brand Contact Lenses 1-Day with HydraLuxe® Technology for ASTIGMATISM.
12. JJV Data on File 2018. Similarities between Mucin and Poly(N-Vinyl Pyrrolidone) (PVP).
13. JJV Data on File 2017. Optical Precision of ACUVUE® OASYS Brand 1-Day Contact Lenses with HydraLuxe® Technology.
14. JJV Data on File 2021. Dual Action Technology and “Cushion of Moisture” Description for 1-DAY ACUVUE® MOIST Brand Family of Contact Lenses.
15. Heynen M, Omali NB, Fadli Z, et al. Selectivity and localization of lysozyme uptake in contemporary hydrogel contact lens materials. J Biomater. Sci. Polym. Ed. 2017;28(13):1351-1364. doi: 10.1080/09205063.2017.1327751.
16. JJV Data on File 2020. ACUVUE® OASYS MULTIFOCAL Fit and Performance Claims.
17. Canavan K, Sulley A, Coles-Brennan C, et al. Multi-Center Clinical Evaluation of Lapsed Wearers Refitted with senofilcon A Contact Lenses. Optom Vis Sci 2014;91:e-abstract 145180.
18. JJV Data on File 2017. A 4-week randomized, masked, controlled, cross-over, dispensing daily wear study with ACUVUE® OASYS vs. ULTRA, n=127 subjects who spent greater than or equal to 8 hours per day using digital devices during the study period.
ACUVUE® Contact Lenses are indicated for vision correction. For detailed product description and safety information, please consult the Instructions for Use on our Johnson & Johnson website www.e-IFU.com.
© Johnson & Johnson Medical Ltd 2024. ACUVUE®, ACUVUE® OASYS MAX 1-Day, ACUVUE® OASYS, ACUVUE® OASYS 1-Day, 1-Day ACUVUE® MOIST, HydraLuxe®, LACREON®, HYDRACLEAR®, TearStable™ and OptiBlue™ are registered trademarks of Johnson & Johnson. 2024PP14491.